
Executive Summary
When caring for oncology patients, multi-disciplinary tumor boards (MDTB) provide the opportunity for multiple specialties to collaboratively discuss diagnoses and stage cancer utilizing a variety of modalities. This allows the entire care team to contribute to staging and to craft individualized treatment plans, achieving the best possible outcomes for every patient affected by cancer. Studies note that the use of MDTB for newly diagnosed malignancies results in improved treatment outcomes, standardization and adherence to best practice guidelines, improvement in decision making and patient satisfaction (Berardi et al., 2020). Greater Baltimore Medical Center (GBMC) has engaged MDTBs for years at the Cancer Institute, with a paper-based methodology. Moving to an electronic MDTB process was initiated to improve clinician collaboration, reduce scheduling and data prep burdens, improve utilization of outcomes-based, nationally recommended stage-specific treatment guidelines, to use real-time documentation to support accreditation and billing needs and include the collaborative insight in the patient chart for future reference.
Prior to January 2020, GBMC’s Malcolm and Sandra Berman Cancer Institute used seven different disease-specific processes to meet best practice guidelines for MDTBs. This required several full-time staff members to coordinate that the patients being reviewed were represented by the correct clinicians/specialties and to collate the required studies/results for educated discourse and recommendations. During the MDTB, multiple formats of data were utilized, such as handwritten notes, Word documents, PowerPoints and radiology imaging portals. This created a disjointed flow of presentation. Team dialogue and treatment plans were noted outside of the EHR and rarely were added to the record for later access, simply being stored in binders for administrative needs. Often, staging information was not contained in the MDTB documentation and was not routinely being captured in discrete, standardize fashion in the EHR.
By innovating the MDTB from a diverse paper process to a single electronic format, specialists leverage the EHR to reduce the workload required to schedule patients for review by simply using a few clicks to drop the patient on the disease-specific MDTB resource. This provides clinicians autonomy to review information in advance of a disease-specific MDTB conference and attend encounters as indicated. Data prep time is greatly reduced, because all diagnostics can be accessed via the patient chart using workflows that are integrated into routine work. Real-time documentation of the group’s evaluation, staging and therapy plans has reduced lag time between MDTB and treatment.
Critical to the success of associating the MDTB to the patient chart was the need to ensure the MDTB encounter, and all documentation completed in conjunction with the collaborative time, could be partitioned within the patient’s chart. This would allow participants the ability to meet documentation requirements for accrediting bodies and bill accurately for time spent, while preventing patients from getting unnecessary appointment alerts. This also allowed for documentation suppression from patient portal access. As a result of migrating the MDTB process to the EHR, GBMC was perfectly situated to move the MDTB conferences to a virtual platform required by the COVID-19 social distancing guidelines without interruption to the outstanding patient care that the Malcolm and Sandra Berman Cancer Institute has been recognized for by a multitude of recognitions, certifications and accreditations by national agencies.
Define the Clinical Problem and Pre-Implementation Performance
GBMC chose to standardize and utilize electronic methodology in the MDTB process across all oncologic diseases as an improvement over paper-based documentation. GBMC selected this project over other clinical projects to adhere to our four aims for better health, better care, least waste and more joy. Better health and better care were achieved by an improved ability to access the MDTB documentation from within the patient’s electronic health record and improved capture of discretely staged cancer diagnoses. This project met aims for least waste and most joy by reducing preparatory work to coordinate MDTBs, removing redundancy related to external documentation of the MDTB treatment plans, and completion of requirements to meet accreditation goals. The stewards for our staging metrics include several bodies: Commission on Cancer (CoC), National Accreditation Program for Breast Centers (NAPBC), National Quality Measures for Breast Centers, Quality Cancer Care Recognizing Excellence (QCP), College of American Pathologists (CAP), Radiation Oncology Practice Accreditation (ROPA) (American College of Surgeons, 2020). Prior to implementation, the Cancer Institute at GBMC received many accolades for the quality of care provided, and maintaining this high level of success was paramount to the implementation of this project. Baseline data was gathered related to number of cases per tumor type scheduled for MDTBs, time spent gathering and collating data for presentation and the number of cases in which staging was completed during the MDTB. Pre-implementation data showed that GBMC’s number of scheduled MDTBs between January 2019 and June 2019 was 642 across 6 tumor types and 78% of MDTBs resulted in staging of cancer.
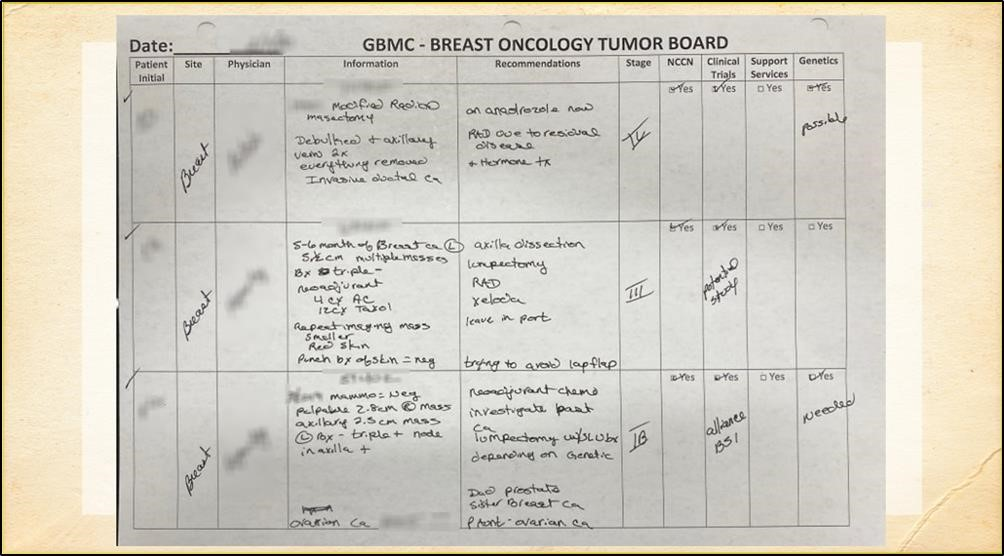
Source: Greater Baltimore Medical Center
As our overall goal, we chose to recognize the benefit of having an integrated MDTB encounter associated in the EHR and contained within the discussed patient’s chart and including a captured staging diagnosis as our measure of success. As the overarching goal, the target of this project was noted as one patient, one digitized record, moving 100% of new case presentations off paper and into the EHR. To quantify the number of MDTBs meeting the goal for EHR needs, we defined the number of MDTB cases scheduled as the denominator population. The number of presented cases resulting in staged outcomes completed as part of the MDTB process, or the application of collective wisdom, were counted as numerator cases. For this change process, no patients or MDTB cases were excluded. As a secondary performance measure, the decrease in clinician and administrative redundant time related to the preparation of case presentation material would be measured by time between scheduled presentation and presentation date, resulting in an inverse metric of success related to decrease in preparation lag times and reduced need for repeated or additional presentations due to lack of requisite data for consensus decision making to form a treatment plan recommendation. The final performance goal was related to the user completing the scheduling of the presentation. In order to reduce administrative support, scheduling case presentations would be moved to the clinical provider. We targeted 100% provider (MD, CRNP, PA) scheduling since the registrars who were doing all the scheduling on paper only have read-only access to the EHR.
Design and Implementation Model Practices and Governance
There are many roles on a MDTB, and developing a tool using integrated work processes to broaden the inclusion of many disparate team members is a significant request. MDTBs at GBMC include oncological providers in medical oncology, radiation oncology and surgical oncology. The Surgical specialties of breast, colorectal, GYN-ONC, thoracic, head and neck, neurosurgery, plastic surgery, pancreatico hepatobiliary and general surgery provide the spectrum of surgical oncology expertise. Likewise, medical specialties in pulmonology, integrative medicine and gastroenterology round out the types of treating providers. Clinicians from pathology, radiology, genetics, physical therapy, rehabilitation, speech, nutrition, lymphedema and dental medicine are available based on the needs of each case. Supportive care providers included in MDTBs represent clinical research, care management, oncology support, various tumor registries, cancer survivorship and social work. Support from the medical director and executive director of the oncology service line and the Cancer Institute was combined with the support of GBMC’s president and chief executive officer to push for reduction of paper processes and incorporating the one patient, one record care continuum.
In addition to the executive and clinical representatives, the project team included the cancer registry administrator, our lead informatics clinician and the oncology analyst from the Epic team. GBMC’s Epic team collated a list of desires for improved functionality for the creation of the project charter and set a five-month timeline from kick-off in August 2019 to Go Live in January 2020. Additional analysts from the scheduling application were charged with the creation of the scheduling template and workflows. The Phase 1 key outcomes were identified in the project charter to include: standardization of the current seven processes across tumor types into one unified process, creation of a single schedule for the conferences with individual resources for each tumor type, ease of access to all aggregated data in the EHR, real-time capture of the MDTB discussion during the case presentation, completion of guideline directed staging forms and incorporation of stage specific oncologic diagnoses into the patient Problem List or Oncological History, and, as a final product, creation of the MDTB note with a national guideline directed recommended treatment plan. The bulk of our project timeline was spent on the analysis and design phases, lasting two months. Meetings with each MDTB region group were conducted to identify key data needed. Build and testing were done concurrently, with the lead informatics clinician and analyst addressing all clinical users’ workflows and contributions to the MDTB. Each user role was tested as background access was added in a layer method to ensure regression testing could be used to identify access problems. End-to-end testing was completed using sample data resulted internally, through interfaces and from patient-entered questionnaires for the Head and Neck symptom management needs. This mimicked the variety of resulted diagnostics used during MDTBs to ensure smooth flow and seamless integration during presentation of cases. End-to-end workflows were also presented for the larger stakeholder group in December 2019 as both demonstration of functionality and a precursor to training. Tip sheets for scheduling and locating the Tumor Board Overview Report were provided as reference materials. One-on-one sessions with clinicians were conducted to increase end-user buy in and address any weaknesses in build as a byproduct of scheduling initial presentations of MDTBs. The lead informatics clinician attended each MDTB in January and February of 2020 to guide usage of the new tool and address any barriers to adoption. A rollout method was used to implement each MDTB, with all tumor types included in electronic presentations by March 2020. In March 2020, the COVID-19 migration to socially distanced tumor boards promoted the quick adoption of the electronic format of virtual MDTBs to ensure patients continued to receive high quality oncologic care with all clinicians contributing to the collective process, regardless of geographic location.

Source: Greater Baltimore Medical Center
Clinical Transformation enabled through Information and Technology
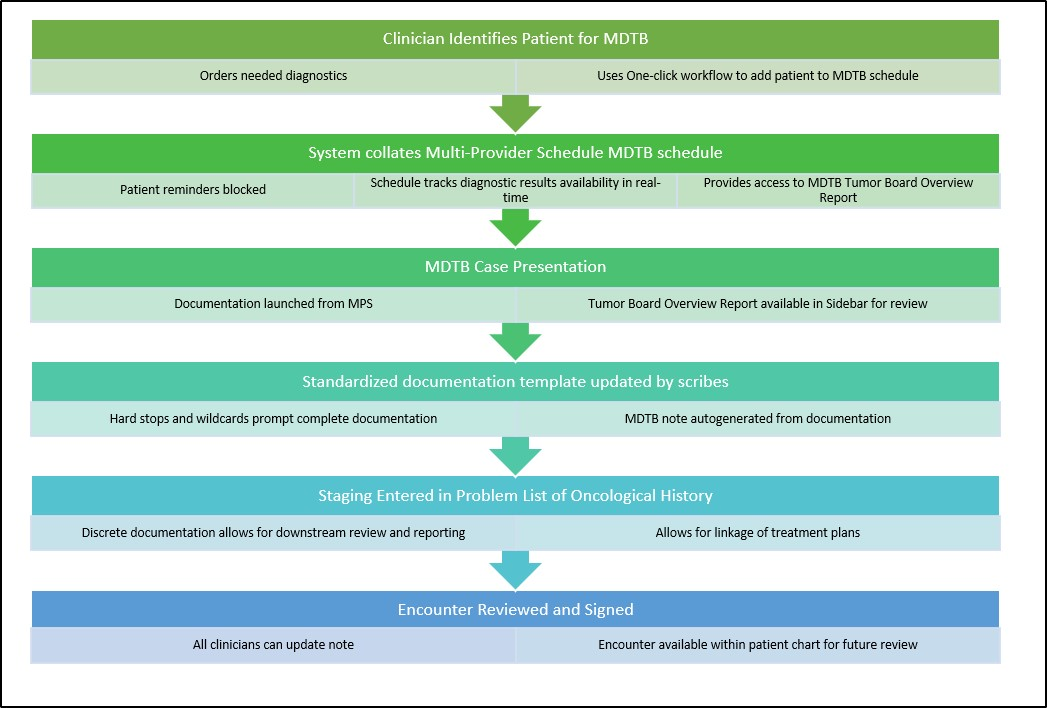
Source: Greater Baltimore Medical Center
Using a sample paper outcome grid, the Epic team was able to recreate the MDTB report as the Tumor Board Overview Report, pulling identified data from patients’ charts without additional effort on the clinicians’ part. Collation of data sources typically used for each region’s MDTB was completed to ensure all needed information was pre-populated into a report for case presentation. These data points included: radiology images and impressions, pathology, cancer specific (e.g. tumor markers) laboratory studies, evaluations and consults of the various team members and recommendations for treatment.
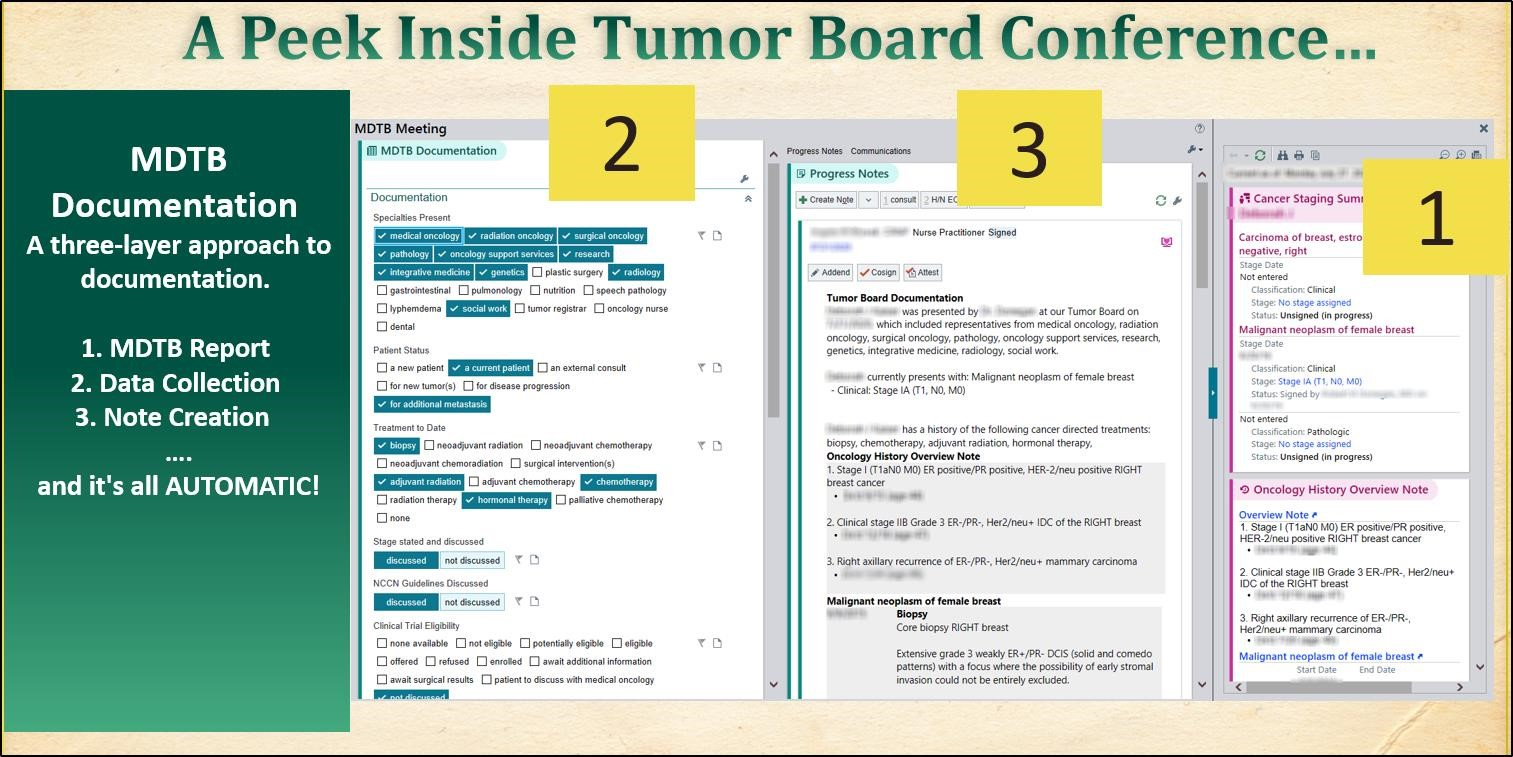
Source: Greater Baltimore Medical Center
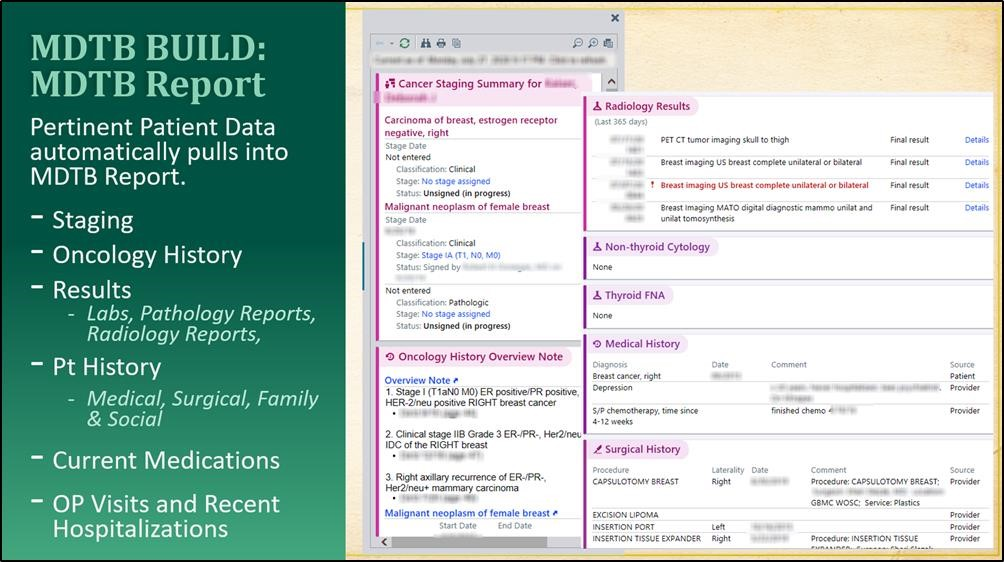
Source: Greater Baltimore Medical Center
During the MDTB case presentation, documentation is completed in real-time by clinical provider “scribes” using simple prompts and a selection of pre-determined responses.

Source: Greater Baltimore Medical Center
This documentation feeds a standard note template automatically for review during the MDTB, which allows updates as needed. Tumor Board Meeting accreditation requirements and clinical operations needs drove the design of the documentation points. Hard stops within the MDTB note prompt the documenting scribe to request clarification of discussion or highlight missing details in real-time. Utilizing the system to produce note efficiencies allowed real-time documentation to reduce the lag time of implementing recommendations of the collaborative treatment plan.
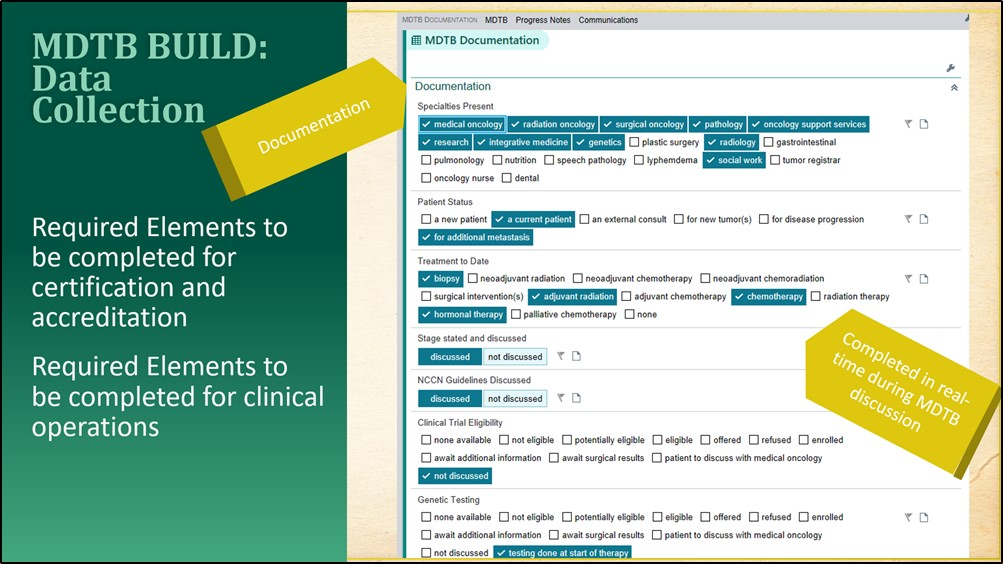
Source: Greater Baltimore Medical Center
Improving Adherence to the Standard of Care
To calculate improving adherence to the stand of care for MDTBs, we defined staging occurrence during the MDTB as the key measure of success. The American College of Surgeons’ Commission on Cancer program requires a minimum of 10% of an institutions annual analytic case load, or up to 200 cases, to be evaluated for several areas, including AJCC Stage or other appropriate staging system based on tumor type or cancer site (2020). Calculation of staging adherence was based on total number of all MDTBs scheduled representing the denominator and completed staging during the MDTB encounter as the numerator population. Pre-implementation, our MDTBs had an average annual staging occurrence of 78%. Post-implementation, that rose to 84%. The significant increase in staged cases, along with the 100% staged head and neck cases signifies an improved process.
Outcome metrics involved in process improvement include:
- The pre- and post-implementation number of scheduled MDTB cases by tumor type (care quality measure)
- The pre- and post-implementation number of cases in which staging was discussed/completed during MDTBs (clinical quality measure)
- Lead time calculated based on date scheduled MDTB entry was made to date of MDTB presentation (reduction of lag from diagnostic results to MDTB)
- Number of scheduled MDTB cases per user, used as a measure of end-user success indicating decreased reliance on support staff
From January to June 2020, GBMC completed 532 MDTBs, compared with 642 from the same timeframe the prior year. This 17% decrease in cases from the pre-implementation timeframe is better than the United States’ decrease of 46.4% for initial cancer diagnoses, related to impact of the COVID-19 pandemic on patients’ access to and consumption of healthcare. The decline in diagnoses was also found in the Netherlands, where a 40% decline was noted, and the United Kingdom, which experienced a 75% drop in referrals of suspected cancer (Kaufman et al., 2020). Bearing that in mind, we chose to focus our evaluation of improvement by percentage of cases with staging completed during the MDTB, the average lead time prior to the MDTB as a measure of prep time required for presentation, and the variety of users scheduling MDTBs to denote the efficiency of clinical user scheduling. Including metrics surrounding lag in preparation to presentation and efficiency of scheduling were included based on recommendations for the improvement of MDTBs published following a global survey for improvement of MDTBs (El Saghir et al., 2015).
Improving Patient Outcomes
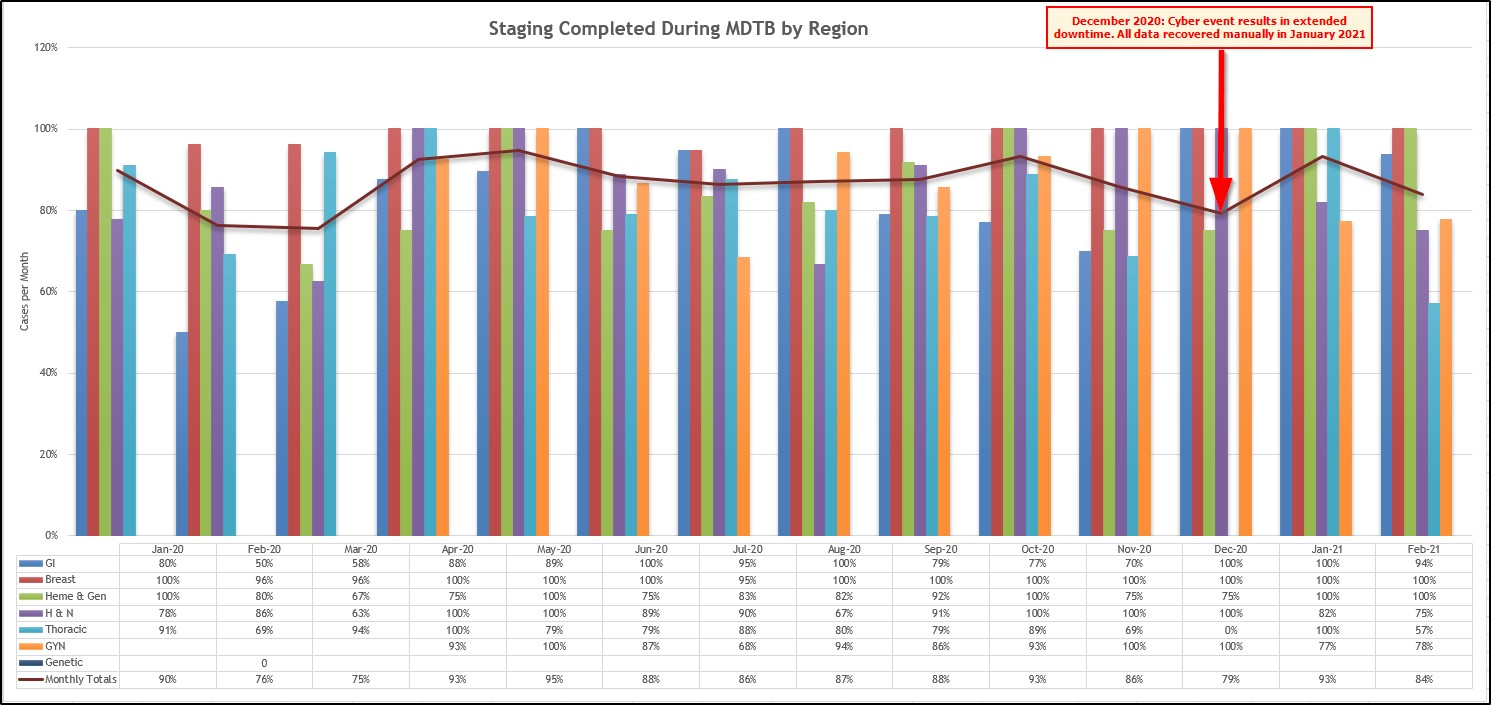
Source: Greater Baltimore Medical Center
For the initial Go Live in January 2020, we used a phased roll-out, with all regions moving to electronic format by April 2020. The slight decline in performance noted in February and March 2020 denotes providers learning the need to add patients to the schedule for presentation. By April 2020, GBMC noted the beginning of our significant improvement timeline. In December 2020, GBMC experienced a significant cyber event, which prevented clinicians from presenting new cases electronically. Despite this extended downtime, once GBMC recovered and was able to return clinical operations using electronic platforms in January 2021, an almost immediate return to an average 102 cases presented per month in the period July 2020 through November 2020 was noted. When calculated over the course of one year, and removing the outlier data for December 2020, January 2021 was on par with the average of 93 presented cases per month. Using the January 2019 to June 2019 average volume of 107 cases presented per month, we are showing improvement over implementation months of January to June 2020 and a near return to prior pre-COVID-19 volumes when compared to April and May 2020.
Patient outcomes when MDTBs are used to discuss individual cases result in better and individualized treatment plans. This prevents delays in care when specialized consults are done independently, especially for complex cancers. The most critical result for patients is that discussion at MDTBs and treatment on national stage-specific treatment protocols improve the chance of survival. Additionally, when the cancer specialists are involved in higher percentages and on a more frequent basis in the discussion, they are more likely to consider new research or emerging treatment options. While GBMC was already conducting a significant number of MDTBs, efficiency was lacking, resulting in a limited volume, failure to communicate the consensus opinion of the MDTB regarding multi-disciplinary care and delays surrounding result tracking. Optimizing the process for MDTBs allowed more patients to be scheduled for the same amount of time and decreased work effort surrounding preparation efforts resulting in a shorter lag between scheduling and completing the MDTB. This change allowed for recommended treatments to be initiated sooner, again improving the patient’s chance of survival. Using the EMR to complete MDTB’s in real-time noted an improvement in full staging by 9% to 87% post-implementation from the baseline of staging in 78% of cases pre-implementation. This discrete documentation of the cancer stage in the EHR meets best practice guidelines because it enables linkage to the most current treatment methodologies. The greatest improvement was the gained by access to MDTB recommendations within the EHR itself, rather than requiring clinicians to visit a centralized storage location to read paper records. Being able to access MDTB notes on demand allows clinicians to quickly review previous treatment plans and support service recommendations while actively seeing patients.
To track burden of preparation, GBMC chose to use the time difference between scheduling a MDTB case presentation and presentation date. Prior to implementation, the average lead time was managed by multiple administrative support staff with significant time pressures to coordinate information from the various diagnostic tests into a PowerPoint presentation. By migrating the review of information to the single source of truth, the EHR, the job of the administrator for preparing for cases was shifted to the EHR and clinical provider. With automatic integration of diagnostic tests and recommendations available, the lead time between scheduling and date of presentation dropped to less than 3 days in 57% of cases presented. In 85% of cases, preparation time was reduced to less than one week. The flexibility of scheduling also allows for scheduled revisions of treatment plans at specific intervals, meaning providers could hold future time slots for review of clinical results of recommended treatment plans by the MDTB following pre-determined guidelines.
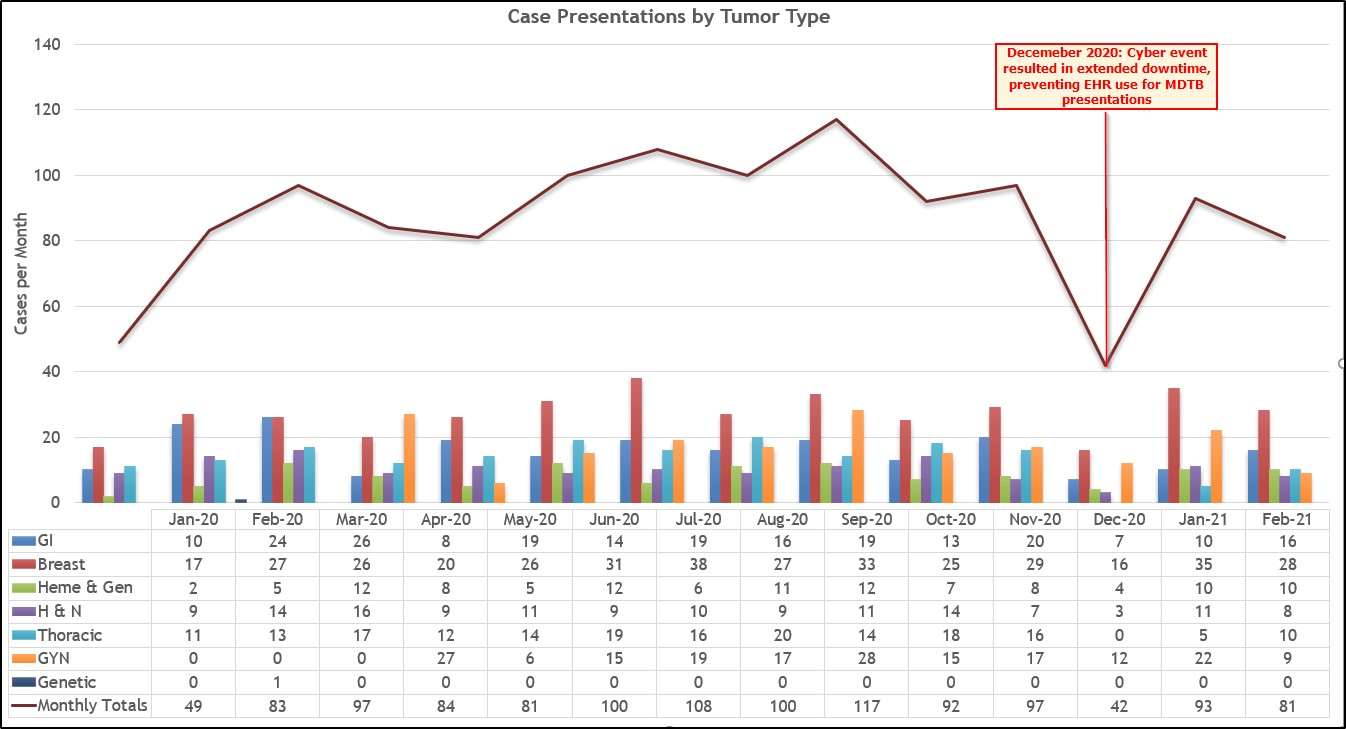
Source: Greater Baltimore Medical Center
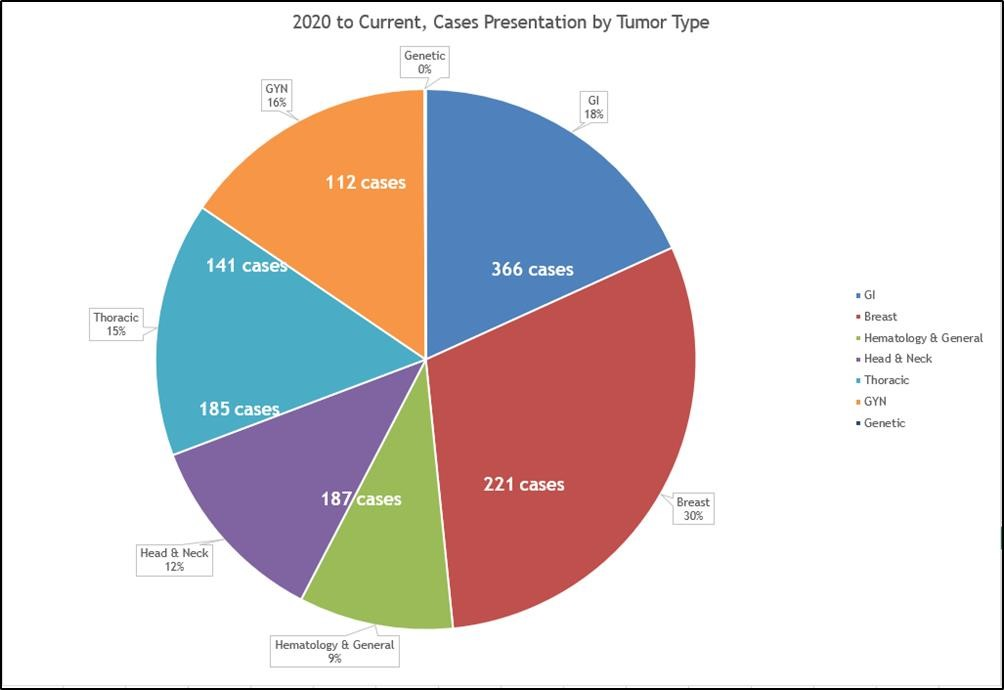
Source: Greater Baltimore Medical Center
Accountability and Driving Resilient Care Redesign
Ease of scheduling presentations, preventing patients from being alerted to the scheduled MDTB encounter and partitioning the MDTB documentation was key to moving the MDTB documentation to the EHR. Goals for the scheduling workflows included reduced reliance on the cancer registry administrator to schedule patients by setting the expectation for treating clinicians to add cases to the MDTB schedule. This reduced the phone calls previously used to ensure necessary representatives were available and results were ready for presentation. A one-click scheduling workflow was implemented for quick scheduling. The generated schedule included a high-level overview of presenter, MDTB tumor type, and diagnostics currently available for review. These columns update in real-time, as ordered tests are resulted, so presenters were aware of diagnostic study status.
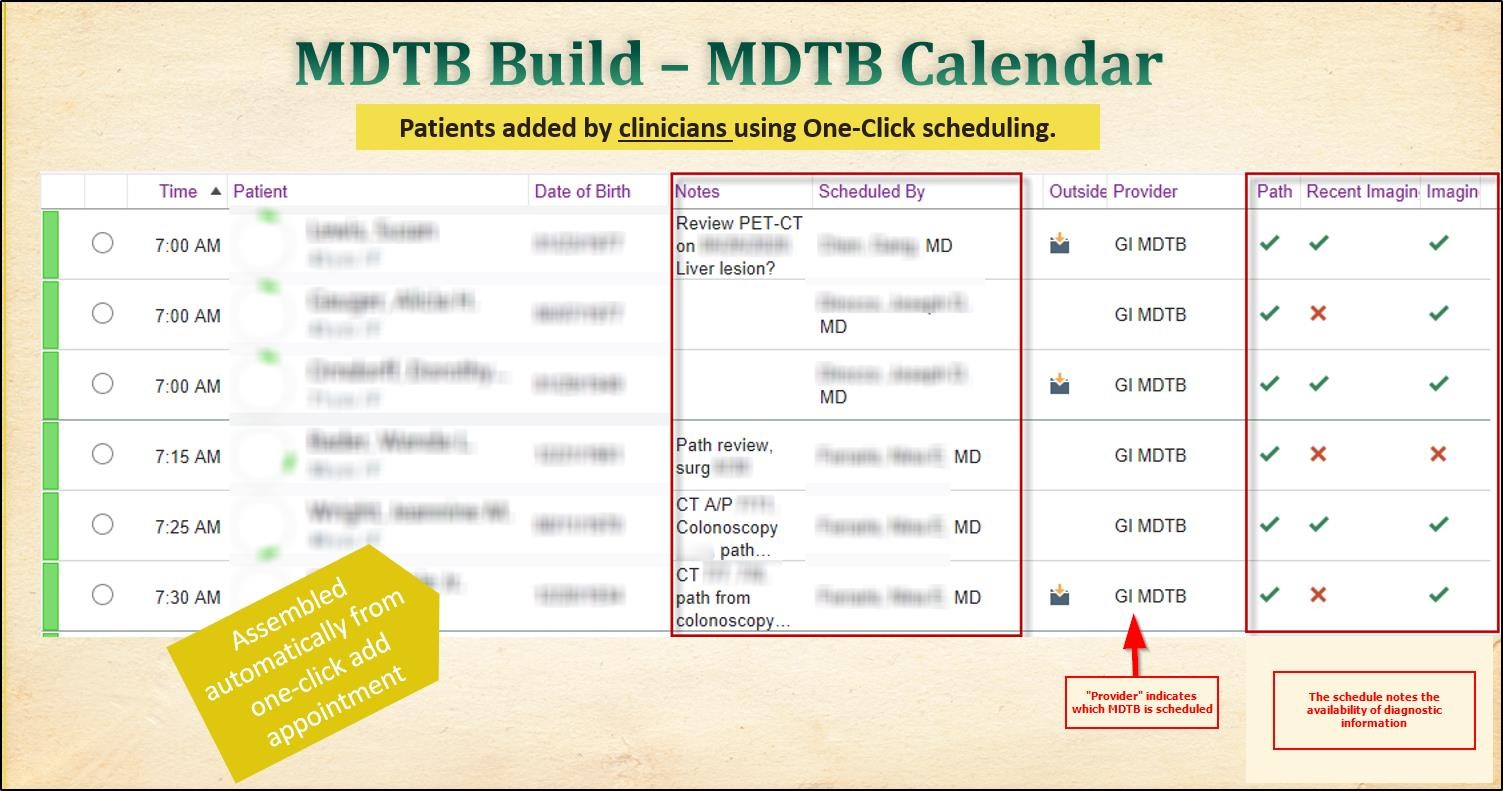
Source: Greater Baltimore Medical Center
By setting the expectation that the MDTB presenter would schedule case presentation, accountability was incorporated into the evolution of the MDTB process. Each clinical user could easily review which provider had scheduled the presentation, and through analytics the project team could track which clinicians were actively engaged in using the new process.

Source: Greater Baltimore Medical Center
To track burden of preparation, GBMC chose to use the time difference between scheduling a MDTB case presentation and presentation date. Prior to implementation, the average lead time was managed by multiple administrative support staff with significant time pressures to coordinate information from the various diagnostic tests into a PowerPoint presentation. By migrating the review of information to the single source of truth, the EHR, the job of the administrator for preparing for cases was shifted to the EHR and clinical provider. With automatic integration of diagnostic tests and recommendations available, the lead time between scheduling and date of presentation dropped to less than three days in 57% of cases presented. In 85% of cases, preparation time was reduced to less than one week. The flexibility of scheduling also allows for scheduled revisions of treatment plans at specific intervals, meaning providers could hold future time slots for review of clinical results of recommended treatment plans by the MDTB following pre-determined guidelines.
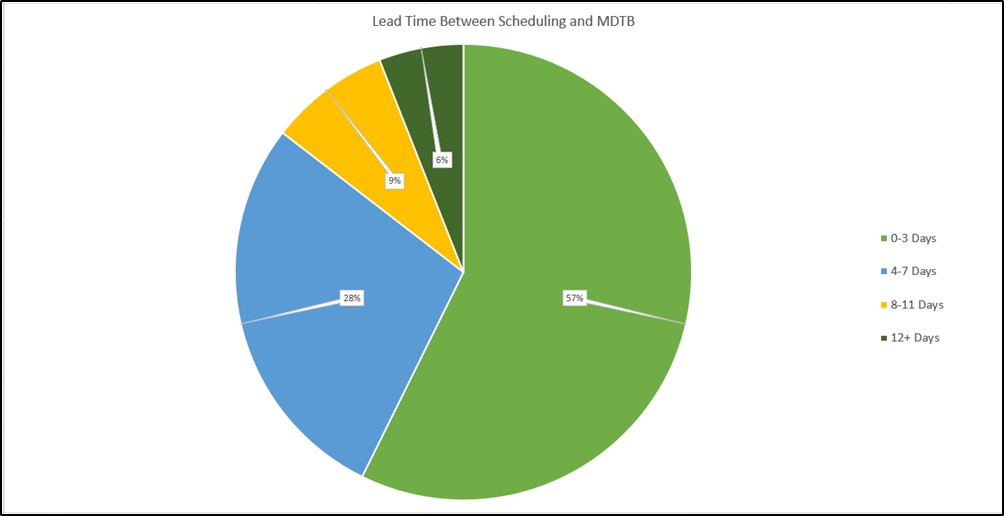
Source: Greater Baltimore Medical Center
References
- American College of Surgeons. (2020). Commission on cancer: Optimal resources for cancer care 2020. Retrieved from https://www.facs.org/qualityprograms/cancer/coc/standards/2020
- Berardi, R., Morgese, F., Rinaldi, S., Torniai, M., Mentrasti, G., Scortichini, L., & Giampieri, R. (2020). Benefits and limitations of a multidisciplinary approach in cancer patient management. Cancer Management and Research, 12, 9363–9374. https://doi.org/10.2147/CMAR.S220976
- El Saghir, N.S., Charara, R.N., Kreidieh, F.Y., Easton, V., Litvin, K., Farhat, R.A., Khoury, K.E., Breidy, J., Tamim, H., Eid, T.A. (2015). Global practice and efficiency of multidisciplinary tumor boards: Results of an American Society of Clinical Oncology International survey. Journal of Global Oncology;1(2):57-64. doi:10.1200/JGO.2015.000158
- Kaufman HW, Chen Z, Niles J, Fesko Y. (2020). Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. The Journal of the American Medical Association Network Open.3(8): e2017267. doi:10.1001/jamanetworkopen.2020.17267
The views and opinions expressed in this content or by commenters are those of the author and do not necessarily reflect the official policy or position of HIMSS or its affiliates.
HIMSS Davies Awards
The HIMSS Davies Award recognizes the thoughtful application of health information and technology to substantially improve clinical care delivery, patient outcomes and population health.



